ECTS: 15-45
Faglige nøgleord: lægemiddelformulering, supramolekylær kemi, fysisk farmaci
Antal Studerende: 1-2
Vejleder: René Holm og Steffen Bähring
Beskrivelse: Cyclodextrins (CDs) are cyclic oligosaccharides, consisting of a variable number of glucopyranose units. The most common applied and investigated CDs are the α-, β- and γ-CDs and derivatives thereof, consist of 6, 7 and 8 of glucopyranose units, respectively. Each of the glucopyranose units is in the rigid chair conformation. The first formulation containing cyclodextrin was market in Europe in 1988 (Scarpignato, 2013) and the first US approval was in 1997 (Crini, 2014). Since its introduction, the number of CD-containing pharmaceutical products approved and marketed has been steadily increasing in a recent review from 2023 the total number of CD-formulated APIs in marketed drug products was reported to be as many as 129 (Puskás et al., 2023), i.e. supramolecular chemistry is well accepted and recognised within the pharmaceutical field and is generally accepted as a safe excipient.
While CDs are a well-established approach, the excipient do not always produce the desired solubilisation capacity for all molecules, why investigations into other supramolecular alternatives may be of relevance. One such alternative is cucurbiturils (CBs). Both CDs and CBs have hydrophobic cavities that accommodate apolar guests by so-called hydrophobic effects driving the complexation process. The macrocycles can be synthesized with different numbers of monomer-units to fine-tune the cavity size, see figure 1.
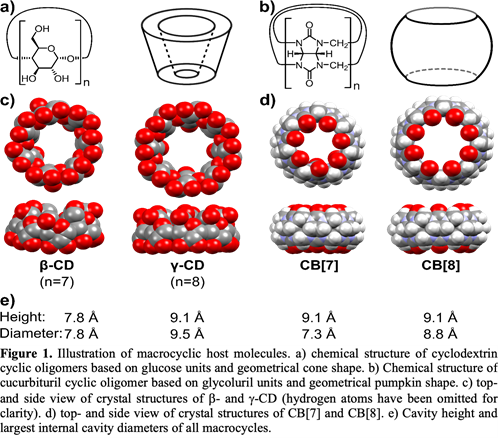
CBs have partially been characterised in the supramolecular literature, however, increased investigations for pharmaceutical applications would be relevant to better understand if the molecules have a place within drug delivery.
The purpose of this project is therefore to investigate the solubility of a range of different drug compounds in different CBs with or without pH adjustments with the use of phase solubility studies and potentially to characterise the interaction thermodynamically by application of ITC to understand the molecular driving forces.
Metoder: phase solubility studies with HPLC or ITC and potentially NMR
Referencer:
Scarpignato C. Piroxicam-β-cyclodextrin: a GI safer piroxicam. Curr Med Chem. 2013;20:2415-2437.
Crini G. Review: a history of cyclodextrins. Chem Rev. 2014 Nov 12;114:10940-10975.
Puskás I, Szente L, Szocs L, Fenyvesi É. Recent list of cyclodextrin-containing drug products. Period Polytech Chem Eng. 2023;67:11–17.