Vejleder: Christine McKenzie og medvejleder Ph.d. Mathias Lander Skavenborg
Projektbeskrivelse
Redox Flow batteries (RFBs) are an alternative to solid state lithium (Li) batteries for large scale energy storage to store energy from windmills or solar panels.[1] In these, the charge carrier is a dissolved redox-active molecule and there is a very large impetus that the solvent in these systems is water, rather than an organic solvent. There are requirements for this energy carrier: very high solubility in water, redox potentials within the redox stability window for water, fast electron transfer, and robustness. The state-of-the-art technology for RFBs is based on simple Vanadium compounds (VRFBs) as the posolyte and negolyte in a cell using VIII/VII|VV/VIV couples. This is elegant, however the problem is that like Li, our planet’s supplies of V are also limited. The discovery of charge carriers based on abundant elements - especially iron since it the most abundant redox active element in the earths’ crust - are highly sought after.
Current Iron RFBs (FeRFBs) rely on simple iron salts and have major shortcomings: H2 evolution on the cathodic side and precipitation or iron oxide on the anodic side of the FeII/Fe0|FeIII/FeII cell. We aim to overcome this by encapsulating the iron atoms in organic ligands. In other words coordination complexes, like you did in the experimental part of KE525. These will keep the iron dissolved and modulate the redox couples such that the aforementioned side reactions are avoided and efficiencies and lifetimes of the FeRFBs are increased.
The project involves the synthesis, spectroscopic, electrochemical and structural characterization of the synthesized charge carriers depicted by the generic charge carrying metal “M” in Figure 1 which illustrates a highly desirable symmetric (or bipolar) redox flow battery.
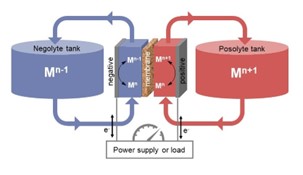
Figure 1. Schematic of a “symmetric” RFB where M is a cheap abundant redox-active transition metal.
The project is part of a large and funded (Det Energiteknologiske Udviklings- og Demonstrationsprogram, EUDP) collaboration with an electrochemistry group at The Technical University of Denmark, Siemens Energy and DFDS (Yes! We want to put iron complexes dissolved in water - i.e., KE525 chemistry- into the hulls of ships instead of oil!).